13 Giugno 2011
English
Formation and appearance of travertine in Tuscany and Latium

Crystalline geode in the travertine deposit of a Serre di Rapolano quarry (ph. Enrico Geminiani).
The oldest Italian standard 1 defines travertine as a calcareous sedimentary rock, a chemical precipitate with a characteristic porous fabric, preferentially used in building and decoration, whereas the most recent European and American standards highlight the complexity of its appearance and characteristics.
According to the European standard 2, travertine is scientifically, a finely crystalline concretionary limestone formed by rapid precipitation of CaCO3 from water; extremely porous or cellular is known as calcareous tufa. Also, translucent, generally layered, cryptocrystalline calcite with coloursin pasted shades, particularly yellow, brown and green formed by slow precipitation in karstic environments.
From a commercial standpoint, it is a natural stone (as described above) that cannot be polished; it can also present itself as “onyx”, a compact banded variety consisting of coloured and transparent layers of calcite or aragonite that takes a polish.

Detail of a travertine from Rapolano Terme.
For the American standard 3 travertine is a crystalline or microcrystalline limestone distinguished by layered structure in which pores and cavities are concentrated in some of the layers, thereby producing an open texture. The material can also be defined as “travertine marble” if it meets the technical requisites specified by the ASTM C503 standard 4 and consists of a layered, porous or spongy, partially crystalline calcite of chemical origin. According to the American standard it is formed by precipitation from generally hot solutions of carbonated spring water, usually at the bottom of shallow pool.
All the standards agree on the chemical-evaporitic origin of the sedimentary rock and on its porous fabric; there is also complete agreement on the denomination “travertine”, the internationally accepted name derived from that used by the ancient Romans to indicate the stone quarried at Tivoli: lapis tiburtinus 5. For more than two thousand years this term of Latin origin has been adopted as the scientific and commercial name of stones throughout the world with the same aesthetic characteristics as the stone from Tivoli, although other local terms may be adopted, for example calcareous tufa in English, tuf calcair in French, Kalktuff in German, or tufa, kankar, sinter. Another interesting basic definition of travertine is found in the exhaustive text by Allan Pentecost entitled “Travertine” 6. According to the author, travertine can be defined as a chemically-precipitated continental limestone consisting of calcite or aragonite formed around seepages, springs and along streams, rivers and, occasionally, in lakes. With a low to moderate intercrystalline porosity and often a high mouldic or structural porosity, it originates within a vadose environment or, occasionally, in a shallow phreatic environment. Precipitation primarily occurs through the transfer of CO2 from or to a source of groundwater that becomes supersaturated in calcium carbonate, with nucleation and growth of crystals occurring in an underwater environment. We could more simply state that it is a concretionary limestone formed thanks to the encrusting power of calcium carbonate dissolved in water. How and why is it possible to find calcium carbonate dissolved in waters? Furthermore, is this element present in all the waters?


Detail of a travertine from Rapolano Terme (foto Enrico Geminiani).
No doubt all the waters in the hydrosphere can act as solvents in the presence of certain salts, and of all the salts that make up rocks, calcium carbonate (CaCO3) is certainly the most soluble. Although the solubility of calcium carbonate is generally equal to 14 mg/litre, it is far from constant. It varies with the concentration of CO2 dissolved in water, the flow pressure of water, water temperature, pH and eH values. Once dissolved in water, carbon dioxide forms an acid (“carbonic acid”) that can attack the limestone in rock masses.
CACO3 +CO2+H2O ? Ca2+ + 2(HCO3)-
Water thus acts as a true solvent, dissolving the carbonate rock within which or above which it flows when, due to a variation in its physical parameters, it becomes supersaturated in calcium carbonate. To re-equilibrate its parameters it precipitates CaCO3, which encrusts all what it touches, forming a layer of travertine.
Waters supersaturated in CaCO3 can form in various ways. For example, the CO2 content of water decreases in the presence of vegetation, causing the precipitation of dissolved CaCO3 (travertine formed from waters at ambient temperature or “meteogene travertine”). Variations in water temperature are also important: a rapid increase in temperature determines an increase in CaCO3 salt concentrations in water, whereas a sudden decrease in temperature causes supersaturation and precipitation.
As in the case of travertine from central Italy, the carbon dioxide present in the water percolating through the carbonate rock mass may also originate through mineralization processes linked to magmatic/volcanic events. Such processes can determine an increase in the dissolution of calcium carbonate which is up to ten-fold that obtained with meteoric carbon dioxide. This phenomenon leads to the formation of widespread travertine deposits (“thermogene travertine”) with a thickness of up to several hundred meters. Their formation can therefore be linked to more or less active volcanism at variable depth.

Sedimentary stratigraphy of a travertine deposit in Rapolano (foto Enrico Geminiani).
Travertine typologies
Travertine is therefore a rock of chemical origin formed through the precipitation of excess CaCO3 dissolved in waters, whose presence may be ascribed to various factors, the most important of which is linked to magmatic activity. The continental environment and conditions in which this carbonate precipitates – waterfalls, running waters, lakes or ponds, springs, sloping mounds, terraced mounds with basins of different size and fissure ridges – along with the type of encrusted organic material, determine the formation of different types of travertine. It is possible to distinguish not only between meteogene and thermogene travertine, but also between autochthonous and allochthonous types. Autochthonous travertine forms through in situ CaCO3 precipitation and encrustation without the subsequent transport of the encrusted material. Depending on the encrusted material and the appearance of travertine, one speaks of autochthonous stromatolitic travertine (superimposed millimetric laminations ascribed to algal or bacterial activity), microhermal travertine (encrusted briophytes generating tiny branched tubular structures) or phytohermal travertine (encrusted microphytes and macrophytes, such as moss and lichens, which form rigid frameworks).
Allochthonous or detrital travertine develops when encrustation occurs on organic or inorganic fragments that can be subsequently transported. They can have different grain sizes and, depending on the kinetic energy of water, may be deposited far from the source of encrusting waters.
Given its process of formation, one can state that travertine is possibly the only material to form quickly in geological time: the continuous flow of supersaturated waters allows the formation of several millimetres of rock per year. Climate factors can affect growth rates: the warm seasons as well as the alternation of day and night favour the deposition of CaCO3, whereas in cold periods, when vegetation is dormant, the decrease in the production of CO2 determines a decrease in the mineralization of water and precipitation of carbonates. Another characteristic aspect of this material is that it begins to evolve as soon as it forms: the organic matter it encrusts quickly decomposes, thereby generating widespread porosity. The salts more soluble than calcium carbonate possibly present in the mass may be dissolved, leading to the formation of secondary porosities, and the percolation of supersaturated waters determines the total or partial recrystallization of primary porosities. This process is responsible for the generally spongy appearance of the upper layers and the normally less porous, more compact nature of the base of the formation.
Colour is one of the most variable characteristics of travertine. Precipitated calcite is white and travertine tends to be of this colour, which can be modified by the presence of coloured pigments. Yellow travertine forms in the presence of limonitic oxides, and red travertine in the presence of hematite. Black travertine forms when magnetite or organic material precipitates along with CaCO3, whereas banded travertine develops due to the presence of different structures and shades of colour.

Detail of a travertine from Viterbo.
Geology
The travertine investigated herein is all thermogenic. Although it derives from different areas and is of variable thickness, its origin is always linked to magmatic activity within the context of Apennine orogenesis, responsible for the more or less intense Cainozoic volcanism throughout the area.
The Apennine Mountains are a structurally complex orogenic belt that began to form in the Late Cretaceous and is still developing to this day. Its evolution 7 can be subdivided into different phases triggered by the migration of the African plate toward the European one, with an initially highly complex setting due to the presence of microplates between the two main ones. Two of these, the Iberia and Adria microplates, have affected Apennine orogenesis the most.
In the Mid-Late Jurassic, the opening of the central Atlantic led to the eastward migration of the African Plate, which veered northward starting in the Late Cretaceous in response to the opening of the North Atlantic Ocean. The Ligurian-Piedmont oceanic basin initially separated Europe and Iberia (at the time joined together), from the African promontory of Adria. About ninety million years ago, a series of break-ups and rotations separated the Iberia and Adria microplates, determining the consumption of the Ligurian-Piedmont Ocean, whereas Africa collided with Europe. As a result, tectonic units were stacked into a pile of increasing height within the area occupied by the present Apennines.
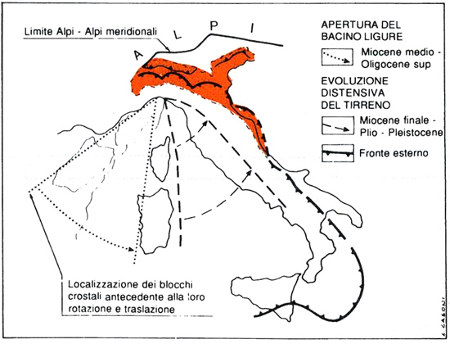
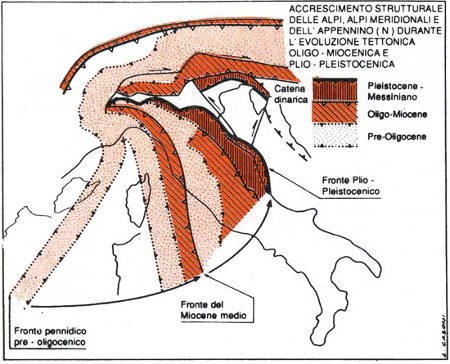
Maps illustrating the rotation and translation of tectonics blocks in the western Mediterranean that led to the formation of the Ligurian-Provencal and Tyrrhenian basins.
Oceanic convergence terminated in the Late Eocene, accompanied by the suture and closure of the Ligurian-Piedmont Ocean, the west-southwestward subduction of the Adria plate below the Iberian plate, and the development of a series of east-northeast trending tectonic processes linked to orogenesis. From the Late Eocene to the present, the Apennine belt developed and evolved in the so-called zone of ensialic convergence, and the belt – foredeep system and undeformed foreland developed adjacent to the present-day Adriatic Sea. The Tyrrhenian Sea is considered to be an extensional back-arc basin characterized by the juxtaposition of slightly raised and lowered structures that recall larger scale horst and graben systems and are aligned in an Apennine direction interrupted by faults. The most important volcanic systems of central Italy developed along these structures. A study of the deep structure of the central Apennines confirms the presence of a thinned crust, with the Moho discontinuity at a depth of some twenty kilometres, and high thermal flows which can locally determine anomalous temperature gradients; for example, northeast of Rome the temperature at a depth of 3 kilometres reaches 150 – 300°C, i.e. it is five times higher than in other regions.
The travertine of central Italy forms within a highly complex, volcanically active geological setting. Areas under extension also experience a series of volcanic events that are not necessarily extrusive nor quiescent; their interaction with infiltrated surface water leads to mineralization processes, interaction with carbonate sediments and the formation of thermal travertine*.
Notes
1 UNI 8458 – Building stone. Terminology and classification.
2 EN 12670 – Terminology of natural stone.
3 ASTM C 119 – Standard definitions of terms relating to natural building stone.
4 ASTM C 503 – Standard specification for marble dimension stone (exterior). The physical requirements listed in table 1 of the standard for defining a travertine as “travertine marble” include water absorption (maximum value of 0.20%), density (minimum value of 2305 kg/m3), compressive strength (minimum value of 52 MPa), breaking load (minimum value of 7 MPa), abrasion resistance (minimum value of 10) and flexural strength (minimum value of 7 MPa).
5 Giorgio Blanco, Dizionario dell’Architettura di Pietra, Rome, Carocci, 1999, pp. 299; Faustino Corsi, Delle Pietre Antiche, Verona, Zusi, 1991, pp. 224 (I ed. 1845); Enrico Dolci (edited by), Il marmo nella civiltà romana. La produzione e il commercio, Conference Proceedings, Carrara, IMM, 1984, pp. 185; Patrizio Pensabene (edited by), Marmi antichi. Problemi di impiego, di restauro e d’identificazione, Rome, L’Erma di Bretschneider, 1993, pp. 255; Mario Pieri, I marmi d’Italia, Milan, Hoepli, 1964, pp. 435; Pieri Mario, Marmologia. Dizionario di marmi e graniti italiani ed esteri, Milan, Hoepli, 1966, pp. 693; Francesco Rodolico, Le pietre delle città d’Italia, Florence, Le Monnier, 1953, pp. 500.
6 Allan Pentecost, Travertine, Berlin, Springer, 2005, p. 16.
7 AA.VV, Guide Geologiche Regionali. Lazio, Società Geologica Italiana (edited by), Milan, Bema, 1998, pp. 377; AA.VV, Guide Geologiche Regionali. Appennino umbro marchigiano, Società Geologica Italiana (edited by), Milan, Bema, 2001, 2 voll.; AA.VV, Guide Geologiche Regionali. Appennino tosco emiliano, Società Geologica Italiana (edited by), Milan, Bema, 2004, pp. 331.
* This post was originally published as part of the chapter “From matter to material. Formation, appearance and characterization of travertine”, in Alfonso Acocella, Davide Turrini (edited by), Sienese travertine, Florence, Alinea, 2010, pp. 303.
